Abstract Authors
Gert Rutger van Lill - Department of Biotechnology, University for the Western Cape
David Adam - Center for Synthethic Microbiology, Max-Planck-Institut for Terrestrial Microbiology
Nicole Paczia - Department of Biochemistry & Synthetic Metabolism, Max-Planck-Institut for Terrestrial Microbiology
Marla Trindade - Institute for Microbial Biotechnology and Metagenomics, University for the Western Cape
Riaan den Haan - Department of Biotechnology, University for the Western Cape
Abstract Description
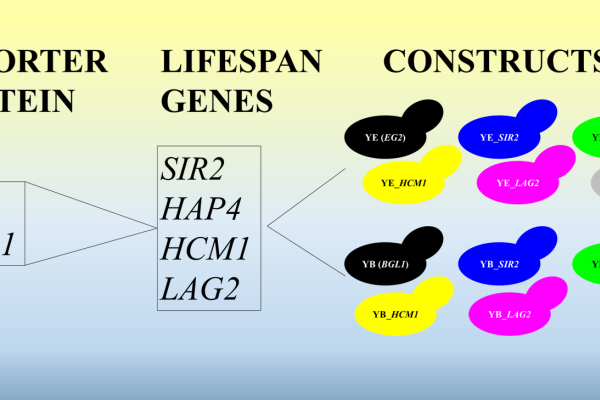
Saccharomyces cerevisiae, is an important host for heterologous expression for industrial purposes, including pharmaceutical, biofuel and high value chemical production. Finding new ways to increase heterologous expression could improve yields, which leads to economic benefits. Yeast, like any other eukaryote, ages. As cells get older, they divide more slowly, accumulate damage, and their metabolic capacity declines. This directly impacts their ability to sustain high levels of protein production. By altering the lifespan of yeast, we aim to maintain an overall physiologically “younger” population for longer. We hypothesize that this would increase overall heterologous expression levels by delaying the decline in cellular performance that normally accompanies ageing. The lifespan can either be increased, thereby rejuvenating the older cells, or decreased, in which case the cells die before reaching older age. Reporter proteins can be used to test how lifespan alteration would affect heterologous expression. Cellulases are industrially relevant proteins important for biofuel production, its activity can be measured using colorimetric assays and they have a great size variety. Thus, they are optimal reporter proteins. S. cerevisiae Y294 was engineered to produce the cellulases endoglucanase and β-glucosidase. The lifespan of these respective strains was increased by overexpressing native S. cerevisiae longevity associated genes SIR2, HAP4, HCM1, or LAG2, respectively, and shortened by knocking out the SIR2 gene. Lifespan alterations were validated using micromanipulation and flow cytometry. The heterologous protein production was assayed using colorimetric assays. Preliminary data confirmed the intended lifespan shifts as expected. Some variants showed an increase in activity while others remained the same. Full datasets and statistical analyses will be presented. Lifespan alteration of S. cerevisiae could be used as a method of increasing heterologous expression




