Abstract Authors
Kedibone Zama Bianca Nene - Division of Medical Microbiology, University of Cape Town
Lynthia Paul - Division of Medical Microbiology, University of Cape Town
Brian Kullin - Division of Medical Virology, University of Cape Town
Abstract Description
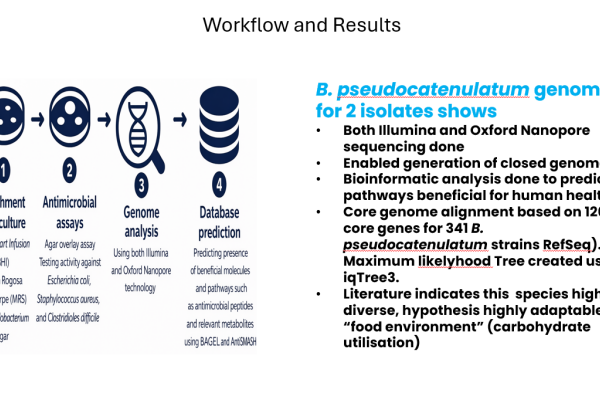
Bifidobacterium pseudocatenulatum is a member of the human gut microbiota, enriched in healthy adults and breastfed infants. It contributes to host metabolic health, immune modulation, and colonization resistance against pathogens. This actinobacterium is garnering increasing interest as a next generation biotherapeutic, due to its metabolic versatility to degrade complex carbohydrates and the production of bioactive metabolites active on the gut-brain axis. Currently this microbe is not part of locally available probiotic preparations, and there is a paucity of knowledge on strains from the South African population. This study aims to isolate diverse Bifidobacterium isolates from faecal donations provided by South African donors. Selective media were used to enrich and culture Bifidobacterium from stored faecal cultures. Antimicrobial assays are done to test activity against various diarrhoeal pathogens. Genome analysis, using both Illumina and Nanopore technology are done on selected organisms to screen for metabolic pathways, virulence traits, etc. Databases such as BAGEL and antiSMASH are used to predict the presence of beneficial molecules and pathways such as antimicrobial peptides and relevant metabolites. Here, we present the results obtained from 2 isolates of B. pseudocatenulatum, obtained from 2 faecal donations. Isolates grown on blood agar lacks hemolytic activity, the genomes indicate the absence of virulence genes. Genome analysis identified a biosynthetic cluster encoding post-translationally modified peptides belonging to the Class IV lanthipeptides. These peptides are predicted to be involved in antimicrobial activity, host-microbe interaction and some antimicrobial activity. The isolates, from two donors, were not close on phylogenetic level, in line with previous studies showing strains from this species being quite diverse compared to other bifidobacteria. Isolation of diverse Bifidobacterium enables expansion of the development of probiotic preparations with diverse benefits to humans. Rigorous genomic screening ensures rational and diverse selection of probiotic candidates, ensuring safety and improved selection of strains with sought after functions, including antimicrobial activity and other impacts on the human body (such as influence on brain and neuronal health). B. pseudocatenulatum represents a promising next generation probiotic, being metabolically diverse, with traits showing ability to adapt in diverse environments and hosts. Its predicted ability to modulate host physiology, degrade complex carbohydrates, and produce neuroactive metabolites underscores its value as potential probiotic for gut-brain axis interventions, and modulation of GIT microbiota using dietary fibre-based microbiome therapies. Future work should include a focus on strain-specific formulation and methods for delivery optimization, clinical validation to harness its full therapeutic potential.

